43 which curve represents the catalyzed reaction?
Answered: This reaction coordinate diagram shows… | bartleby Which represents the catalyzed reaction? O the top curve O the bottom curve This reaction coordinate diagram shows curves for the uncatalyzed and catalyzed mechanistic pathways for the decomposition of H202. PDF CHAPTER 17 STUDY GUIDE ReactionKinetics One curve represents the energy profile for the uncatalyzed reaction, and the other curve represents the energy profile for the catalyzed reaction. ('ourse ofre;lcliml---'J> Aa. Which curve has the greater activation energy? (a)curve 1 (b)curve 2 (c)Both are equal. _-=~"-b. Which curve has the greater energy change, 11K? (a)curve 1 (b)curve 2 ...
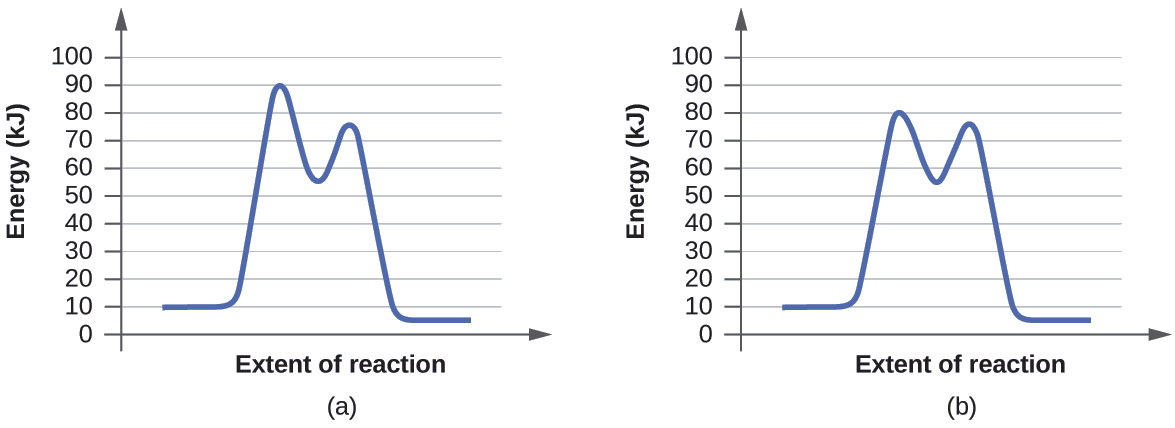
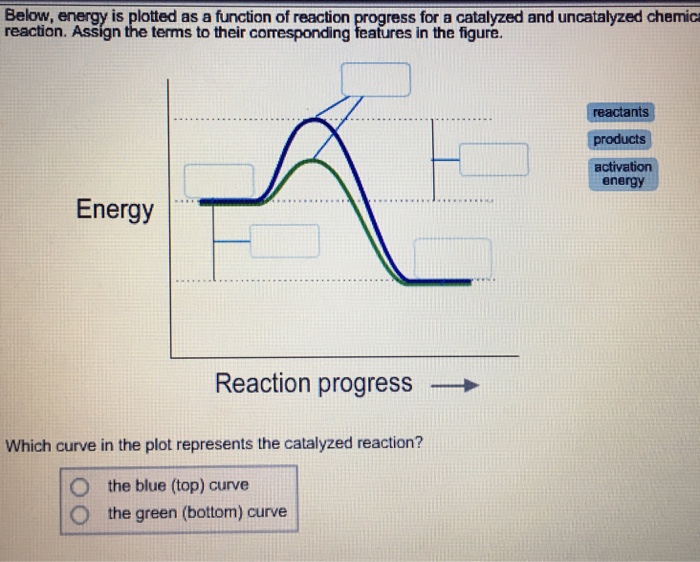
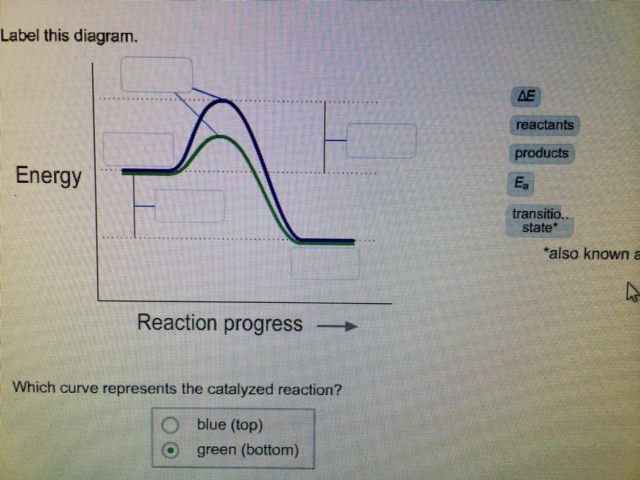
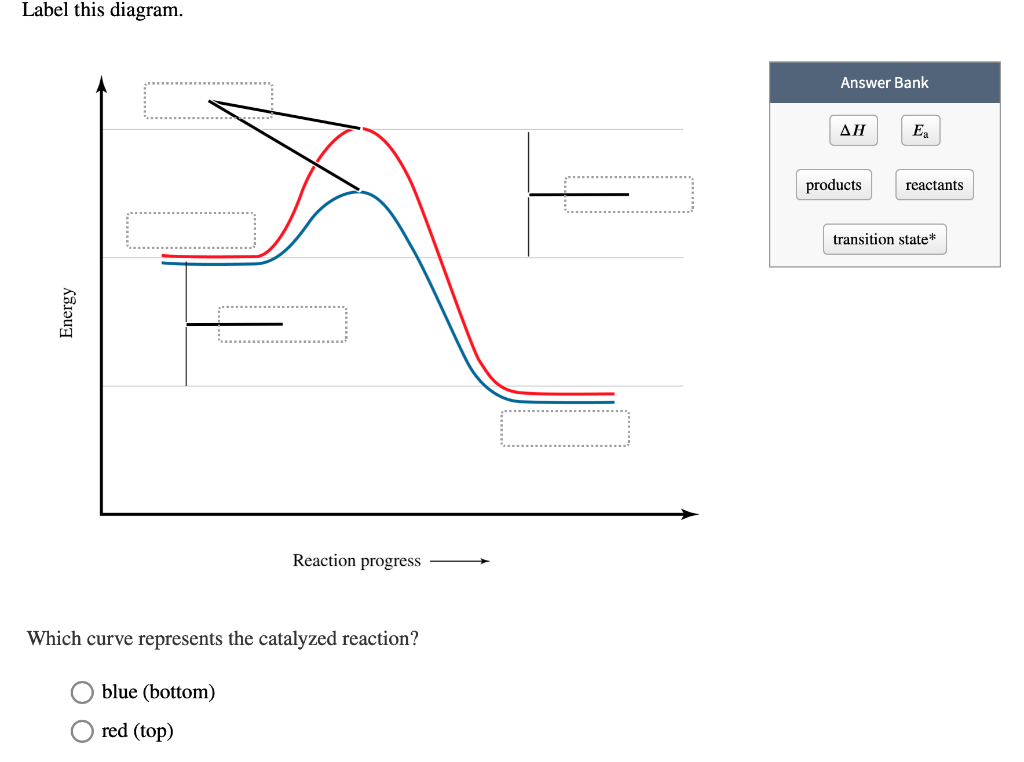
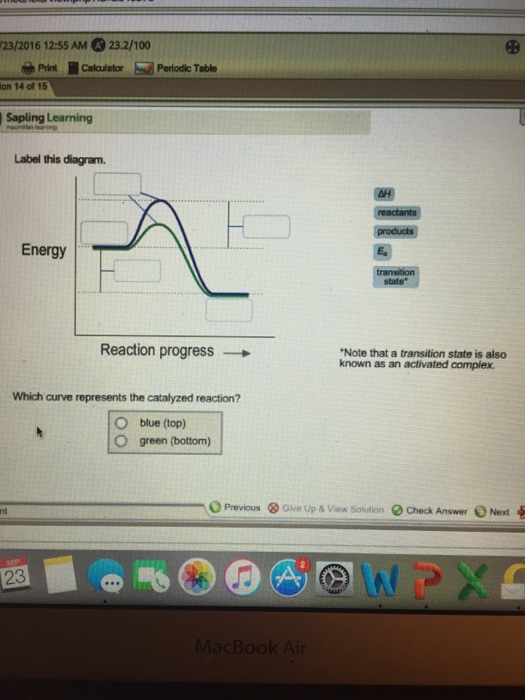
Label this diagram. Energy Reaction progress Which curve represents the ... Energy Reaction progress Which curve represents the catalyzed reaction? O blue (top) O green (bottom) transition state reactants products *Note that a transition state is also known as an activated complex.
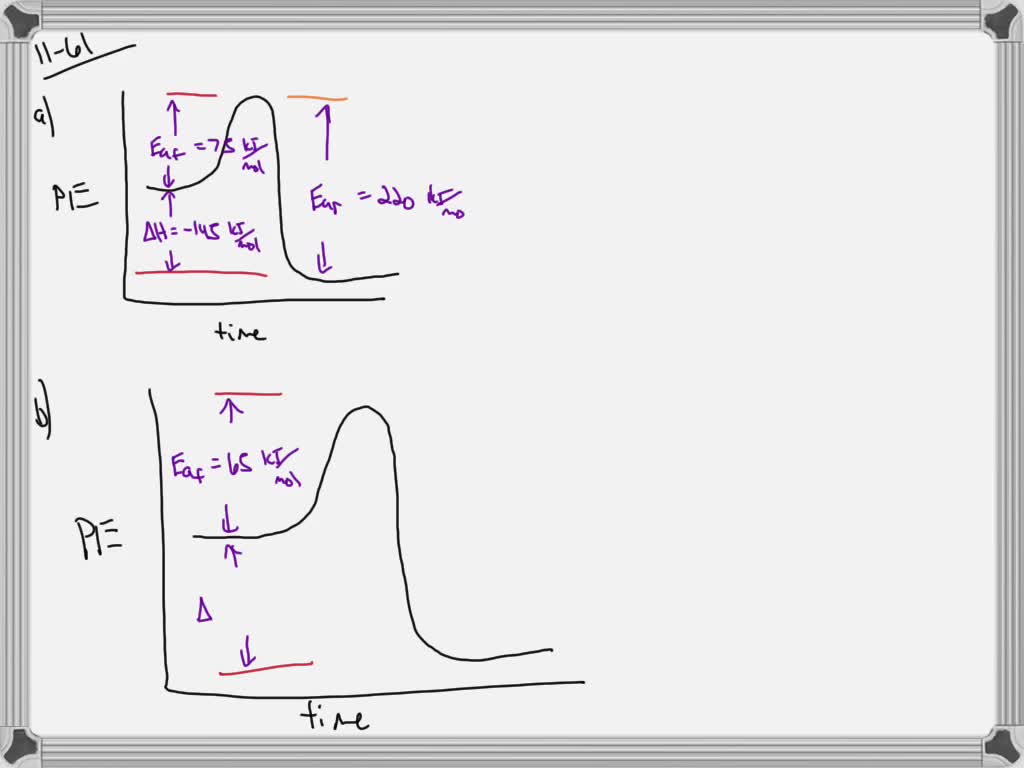
Which curve represents the catalyzed reaction?
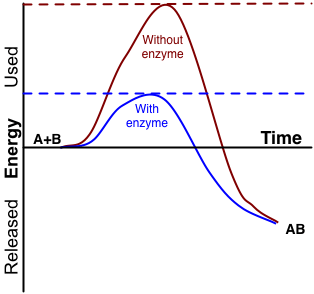
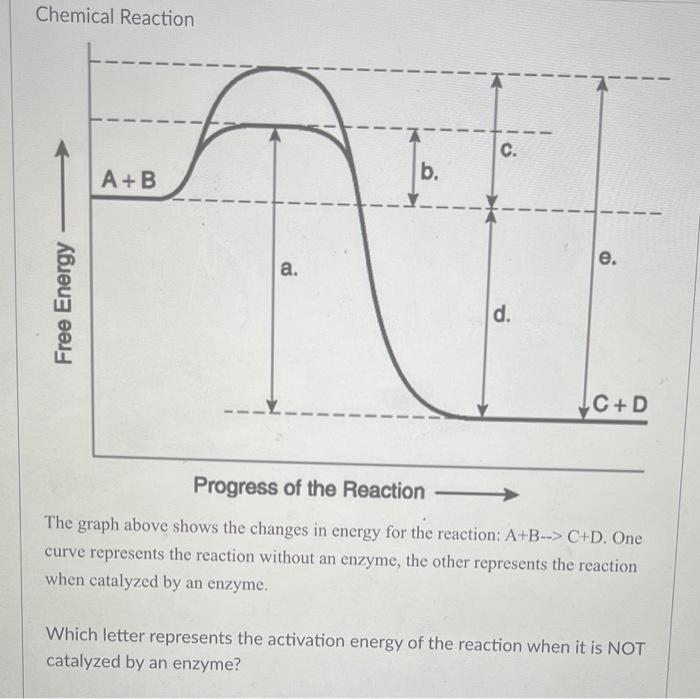
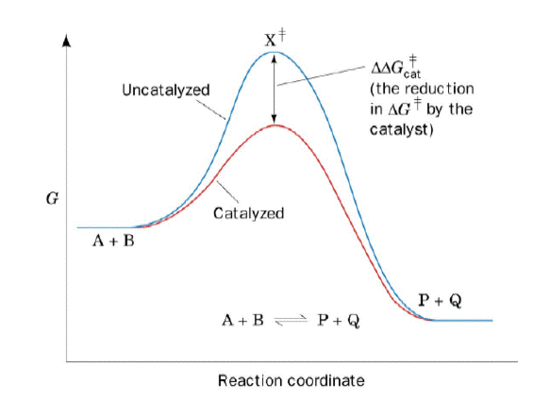
Chapter 5: Quiz 3 Flashcards | Quizlet The answer to this dilemma lies in _____ - molecules that function as biological catalysts, increasing the rate of a reactions without being consumed by the reaction. The lower (red) curve. Line a is the activation energy without enzyme; b is the activation energy with enzyme; c is the change in energy between reactants and products, which is the same for both the catalyzed and uncatalyzed reactions. Answered: 1.) Consider the following reaction… | bartleby Science Chemistry Q&A Library 1.) Consider the following reaction coordinate diagram of the same reaction. One curve represents the uncatalyzed and the other the catalyzed reaction. PDF CHAPTER 13 | Chemical Kinetics: Clearing the Air - WOU the left-hand side of the graph) and the transition state (the highest point on the reaction profile curve). Solve Reaction profile (b) has ... Figure P13.12best represents the reaction profile of the catalyzed reaction because the largest activation (c) energy barrier of the catalyzed reaction is lower than the energy barrier of the ...
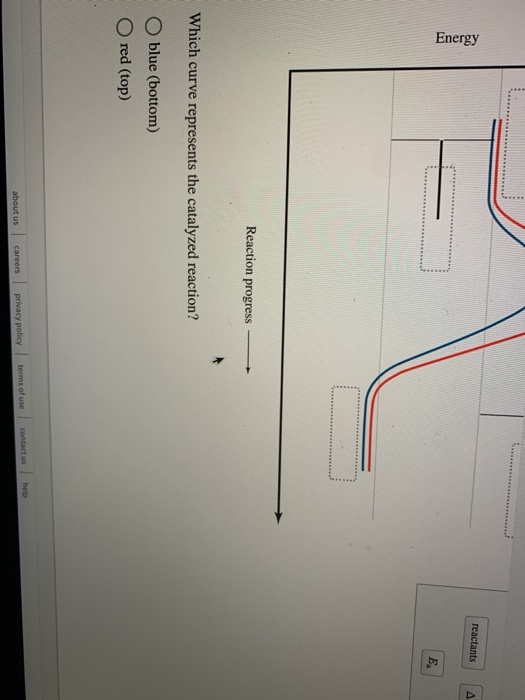
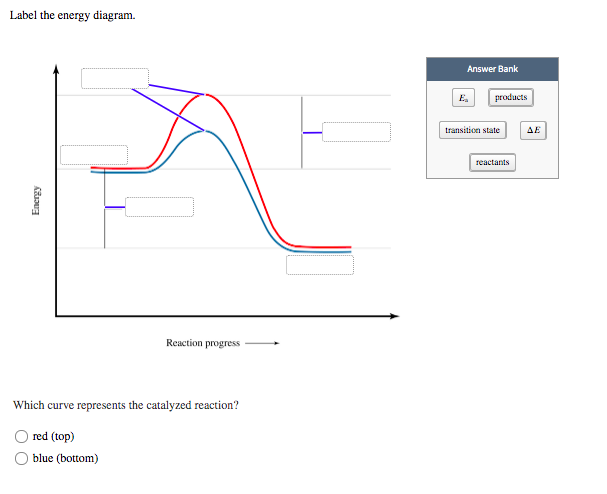
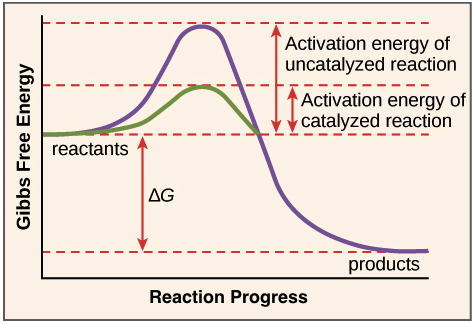
Which curve represents the catalyzed reaction?. Solved Label the energy diagram. Answer Bank E products ... Answer Bank E products transition state reactants Energy Reaction progress Which curve represents the catalyzed reaction? red (top) blue (bottom) Glycolysis: steps, enzymes, and products - Tuscany Diet 06.02.2018 · In most cells this reaction is catalyzed by hexokinase (EC 2.7.1.1), enzyme present in the cells of all organisms, and in humans with four isozyme). Hexokinase and pyruvate kinase, the other kinase of the glycolysis, like many other kinases, require the presence of magnesium ion, Mg 2+ , or of another bivalent metal ion such as manganese, Mn 2+ , for their activity. The diagram below represents a spontaneous reaction (deltaG degree < 0 ... The blue curve represents the uncatalyzed reaction and the green curve represents the catalyzed reaction. Drag the labels in the diagram as follows: Ans: 0 0. Add a comment. ... Select one: O A. Activation energy of the reverse catalyzed reaction o B. Energy difference between the catalyzed and uncatalyzed reactions OC. Activation energy of the ... CTQ7 Flashcards | Quizlet The curve of the graph tells us that a. the change in free energy of both reactions is negative. b. the change in free energy of both reactions is positive. c. the catalyzed reaction has a greater amount of free energy than the uncatalyzed reaction. d. the change in free energy is the same for both reactions. e. Both a and d
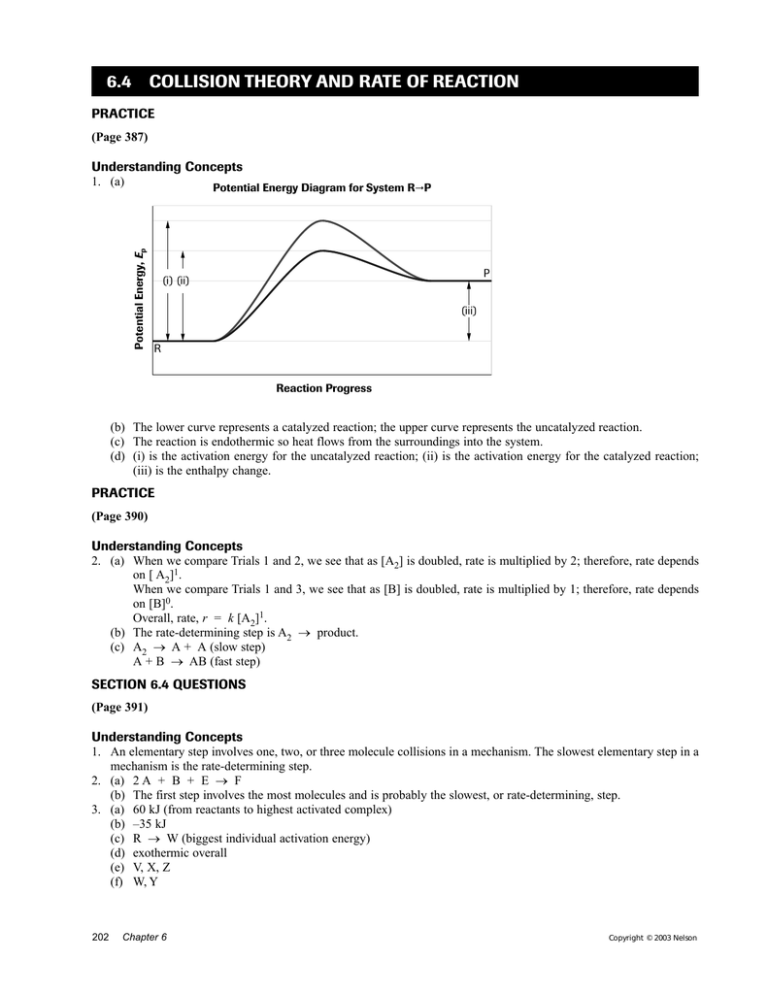
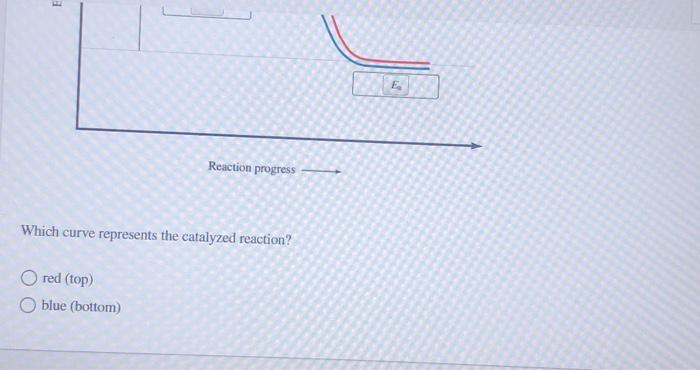
PDF Graph Review - ISD 622 1. Below is an energy diagram for a particular process. One curve represents the energy profile for the uncatalyzed reaction, and the other curve represents the energy profile for the catalyzed reaction. Course reaction a. N%ich curve has the geater activation energy? Curve 1 or cunre 2? b. WIIich curve has the greater energy chanoe, AE? b)curve 2 Solved Label this diagram Which curve represents the - Chegg Science. Chemistry. Chemistry questions and answers. Label this diagram Which curve represents the catalyzed reaction? Blue (top) green (bottom) Progress curve for enzyme-catalyzed reaction - Big Chemical Encyclopedia Figure 4-6 Progress curve for a catalyzed reaction where the initial reaciant (substrate) concentration, [S]o, is significantly greater than the initial enzyme concentration, [E],. As the ratio of [S]o/[E]i increases, the steady-state region accounts for an increasing fraction of the total reaction time. T represents the presteady-state interval. Solved Below, energy is plotted as a function of reaction ... n. products reactants activation energy Energy Reaction progress Which curve in the plot represents the catalyzed reaction? the blue (top) curve the green (bottom) curve O ; Question: Below, energy is plotted as a function of reaction progress for a catalyzed and uncatalyzed chemical reaction. Assign the terms to their corresponding features in the figure.
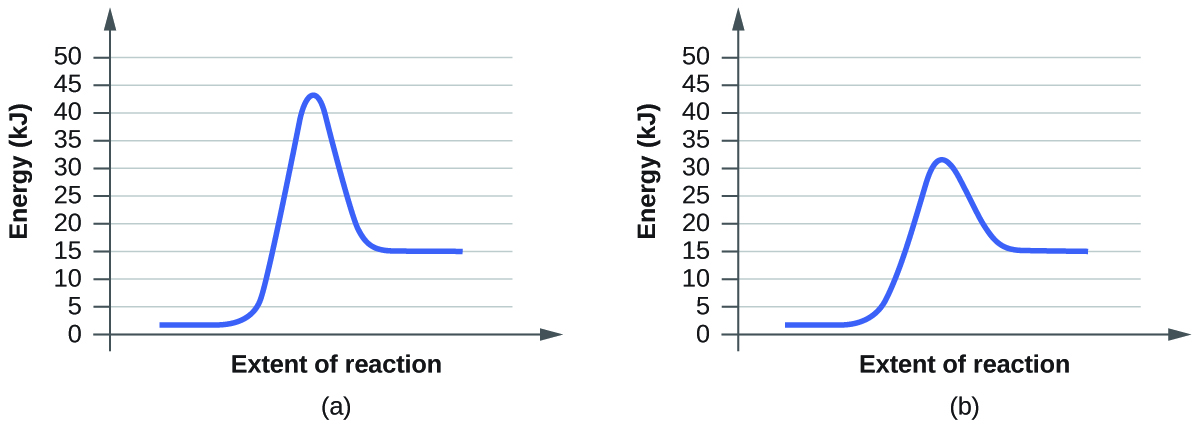
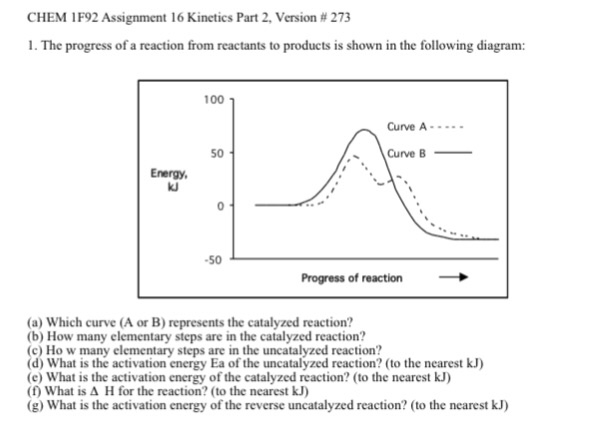
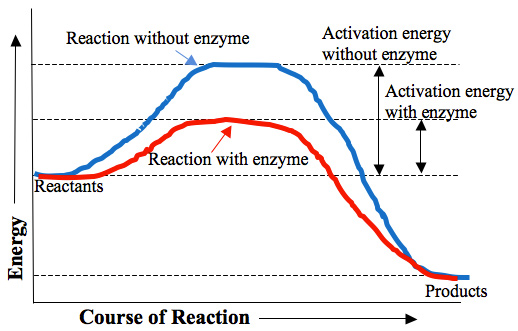
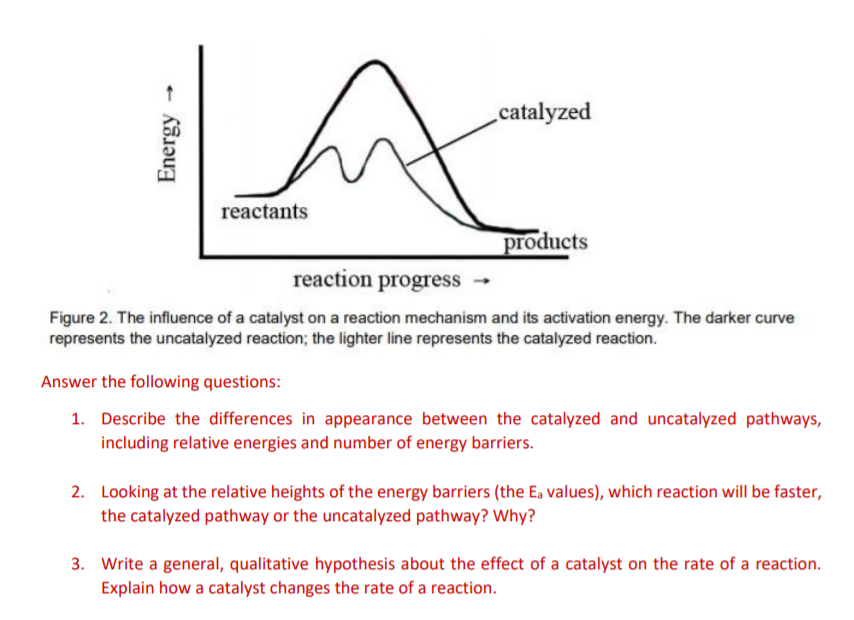
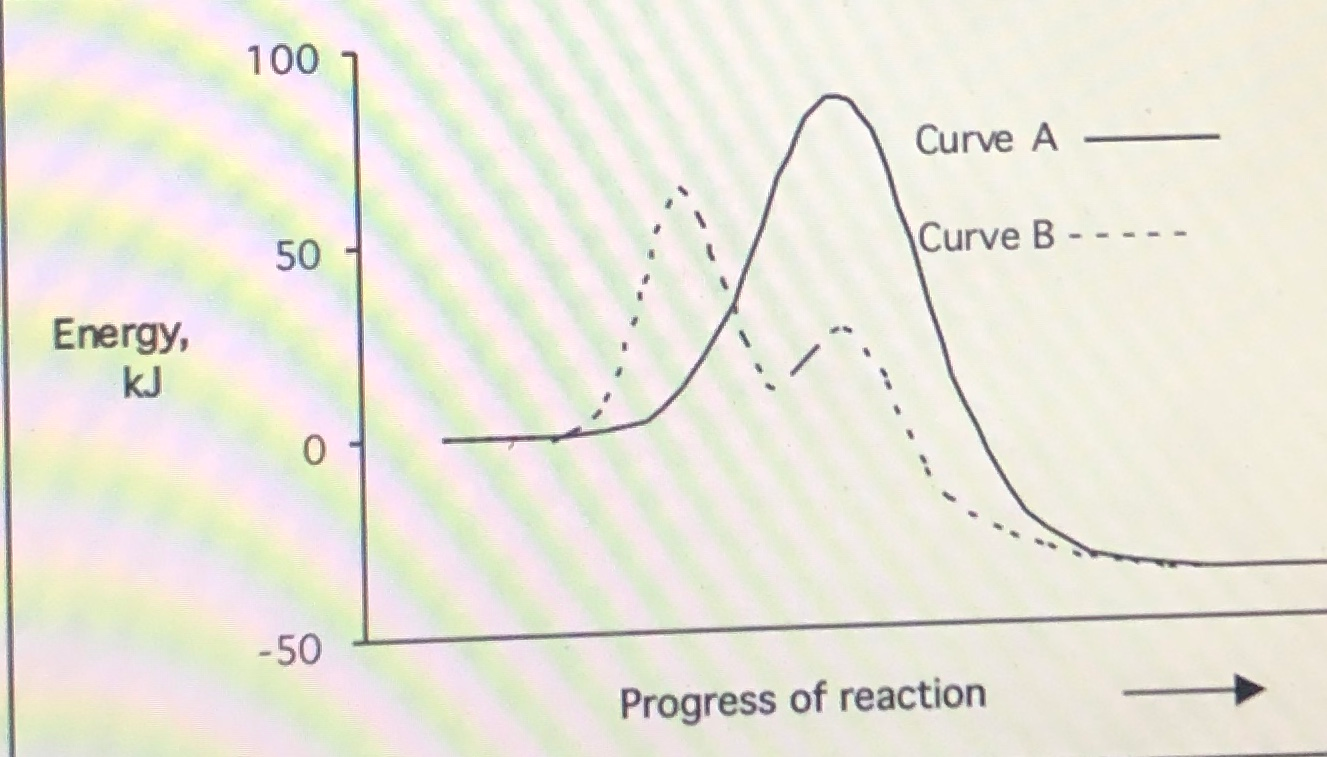
Label the following diagram. Which curve represents the catalyzed reaction? it lã Kineues Part 2, version # 272 1. The progress of a reaction from reactants to products is shown in the following diagram: 100 1 Curve A - 50 Curve B- kuJ -50 (a) Which curve (A or B) represents the catalyzed reaction? (b) How many elementary steps are in the catalyzed reaction? (c) Ho w many elementary steps are in the uncatalyzed reaction? Which diagram shows the effect of catalysis on chemical equilibrium? Your Figure 2 is correct if we want the y-axis to represent a rate-of-conversion instead, i.e., if the upper curve is "number of parcels of $\mathrm{A+B}$ being converted to $\mathrm{C+D}$ per second", and the lower curve is vice-versa. 12.7 Catalysis - Chemistry 2e | OpenStax Figure 12.19 Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. The catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two transitions states). Section 13.3: The Rate of a Reaction - University of Illinois Urbana ... The screened area under each curve represents the number of molecules at that temperature with an energy greater than A. The screened area is much larger under the higher-temperature curve. Therefore, at the higher temperature, more collisions occur and the reaction proceeds faster. ... The reaction is catalyzed by iodide ion. The equation for ...
The role of adsorbed hydroxide in hydrogen evolution reaction … 19.10.2020 · The bifunctional mechanism that involves adsorbed hydroxide in the alkaline hydrogen oxidation and evolution reactions, important in hydrogen fuel cells and water electrolysers, is hotly debated.
Which curve represents the catalyzed reaction?; . ...
Reaction coordinate - Wikipedia In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities.In molecular dynamics simulations, a reaction coordinate is called collective variable.. These coordinates can sometimes represent a real …
Study on co-pyrolysis and products of Chlorella ... - ScienceDirect The residual pyrolysis stage was corresponding to the area from the trough behind the highest peak to the end of the reaction in the weight loss rate curve. In this stage, some substances with high thermal stability were decomposed, while the pyrolysis residue also cracked slowly. 3.2.1. Effect of a single additive on the co-pyrolysis characteristic. Fig. 2(a), (e) and Fig. 3(a), (e) …
The progress of a reaction from reactants to products is... View A16.pdf from CHEM 1P92 at Brock University. CHEM 1F92 Assignment 16 Kinetics Part 2, Version # 235 1. The progress of a reaction from reactants to products is shown in the following
Ambient-pressure synthesis of ethylene glycol catalyzed by 14.04.2022 · Ethylene glycol (EG) is commonly used as antifreeze and feedstock for polyethylene terephthalate used in bottles and packaging ().In contrast to the production of petroleum-derived EG (), EG can also be produced from syngas (CO and, more recently, CO 2 mixed with H 2) (3, 4).Direct hydrogenation of syngas toward EG requires high pressure (>100 bar) at 230°C but …
Determining How a Catalyst Increases Rate of a Reaction by Lowering the ... Curve A has higher activation energy than the original reaction, so it can't represent the catalyzed reaction. Curve C appears to have 0 activation energy, which is impossible even with a catalyst ...
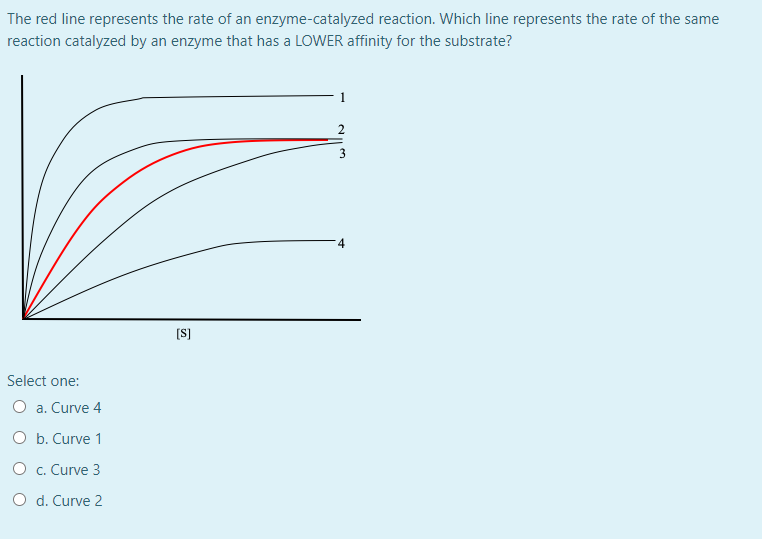
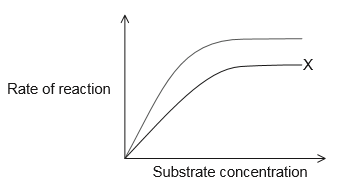
QUIZ 2 mega quizlet Flashcards | Quizlet The dashed green curve represents the enzyme in the presence of the effector. R is the highly active form of the enzyme and T is the less active form of the enzyme. Assume that this is a positively cooperative enzyme, meaning that the affinity for substrate increases with increasing substrate concentration.
Answered: Which curve represents the spontaneous… | bartleby Solution for Which curve represents the spontaneous reaction, and which the nonspontaneous?
PDF Isd 622 uncatalyzed reaction, and the other curve represents the energy profile for the catalyzed reaction. Course of a. Which curve has the greater activation energy. Curve 1 curve 2? b. Which curve has the greater energy change, AE? (a)curve 1 b)curve 2 (c) 0th are equal. b)curve 2 c. VThich curve represents the catalyzed process? (a) curve 1 5.
Protein Function - Molecular Biology of the Cell - NCBI Bookshelf The loss of a phosphate group occurs when the bound GTP is hydrolyzed to GDP in a reaction catalyzed by the protein itself, and in its GDP bound state the protein is inactive. In this way, GTP-binding proteins act as on–off switches whose activity is determined by the presence or absence of an additional phosphate on a bound GDP molecule ( Figure 3-70 ).
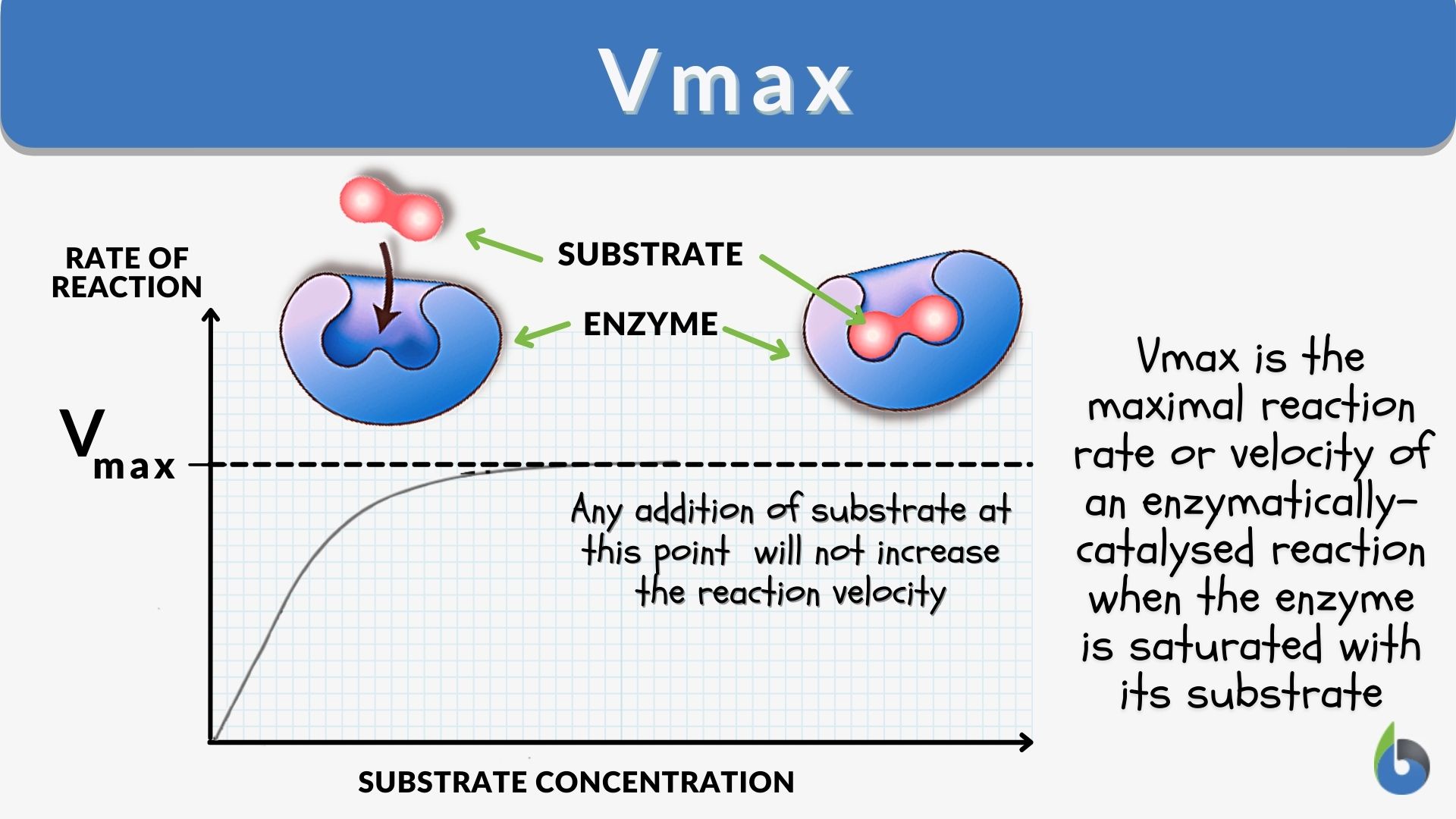
Chapter 7 Flashcards | Quizlet A) enzymes show very little specificity for their substrates. B) enzymes catalyze reactions in only one direction. C) enzyme activities can often be regulated. D) enzymes reaction rates are generally slower than other chemical catalysts. E) enzymes operate under a wide range of temperatures and pH. C.
PDF 17 Reaction Kinetics - SharpSchool Below is an energy diagram for a particular process. One curve represents the energy profile for the uncatalyzed reaction, and the other curve represents the energy profile for the catalyzed reaction. a a. Which curve has the greater activation energy? (a) curve 1 (b) curve 2 (c) Both are equal. c b. Which curve has the greater energy change, E? (a) curve 1
Chemistry selected topics Flashcards | Quizlet the catalyzed reaction. Which curve has the greater activation energy? ... Which curve represents the catalyzed process? Explain your answer to part c. (a)curve 1(higher) (c)Both are equal. (same start and end points) (b)curve 2(shorter) The catalyst forms an alternative activated complex that requires a lower activation
Label this diagram Which curve represents the catalyzed reaction? Blue ... label this diagram Label this diagram Which curve represents the catalyzed reaction? Blue(top) green (bottom)
PCR Amplification | An Introduction to PCR Methods | Promega Once assembled, the reaction is placed in a thermal cycler, an instrument that subjects the reaction to a series of different temperatures for set amounts of time. This series of temperature and time adjustments is referred to as one cycle of amplification. Each PCR cycle theoretically doubles the amount of targeted sequence (amplicon) in the reaction. Ten cycles theoretically …
Catalysis | Chemistry for Majors - Lumen Learning Figure 1. Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. The catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two transitions states).
PDF CHAPTER 13 | Chemical Kinetics: Clearing the Air - WOU the left-hand side of the graph) and the transition state (the highest point on the reaction profile curve). Solve Reaction profile (b) has ... Figure P13.12best represents the reaction profile of the catalyzed reaction because the largest activation (c) energy barrier of the catalyzed reaction is lower than the energy barrier of the ...
Answered: 1.) Consider the following reaction… | bartleby Science Chemistry Q&A Library 1.) Consider the following reaction coordinate diagram of the same reaction. One curve represents the uncatalyzed and the other the catalyzed reaction.
Chapter 5: Quiz 3 Flashcards | Quizlet The answer to this dilemma lies in _____ - molecules that function as biological catalysts, increasing the rate of a reactions without being consumed by the reaction. The lower (red) curve. Line a is the activation energy without enzyme; b is the activation energy with enzyme; c is the change in energy between reactants and products, which is the same for both the catalyzed and uncatalyzed reactions.











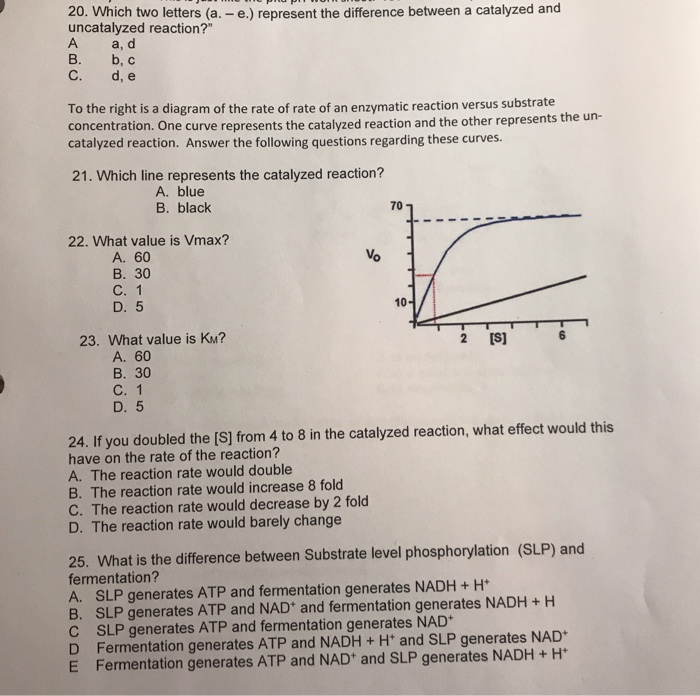
Post a Comment for "43 which curve represents the catalyzed reaction?"